N95 Mask Certification Guide: NIOSH and FDA
Since the COVID-19, many companies and the populace are concerned about N95 masks, and this article talks to you about the certification of N95 masks. First, let’s take a look at the U.S. regulatory body for masks (respirators).
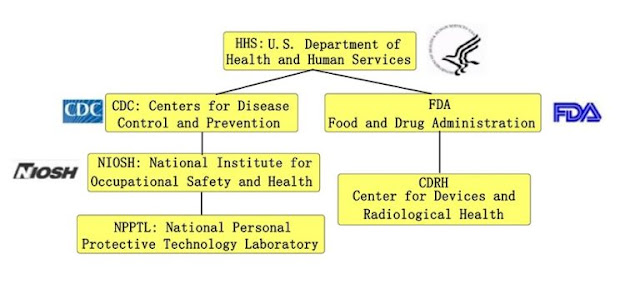
In simple terms, NIOSH is responsible for the certification of personal protective respirator N95, FDA is responsible for the registration audit of medical masks, and medical N95 masks are certified and registered by two agencies.
NIOSH Certification Authority
N95 is called Respirator in English, now it is called N95 mask in COVID-19, which is actually very different from ordinary masks.
The National Institute for Occupational Safety and Health (NIOSH) and the National Personal Protective Technology Laboratory (NPPTL) are the agencies specifically responsible for respirator product certification.
NRP Series
According to the US Federal Code 42 CFR part 84, NIOSH classifies anti-particle masks into 3 series of 9 categories.
| Classification | N series | R series | P series |
| Features | Only filter non-oily particles. | Filters both oily and non-oily particulate matter, but cannot filter oily particulate matter for more than 8 hours. | Filters both oily and non-oily particulate matter. |
| Filtration efficiency ≥ 95% | N95 | R95 | P95 |
| Filtration efficiency ≥ 99% | N99 | R99 | P99 |
| Filtration efficiency ≥ 99.97% | N100 | R100 | P100 |
“N95” means 95% filtration efficiency under the testing conditions specified in the NIOSH standard, N95 is not a specific product name and “N” does not stand for “N” in NIOSH. As long as the product meets the N95 standard and passes the NIOSH review, it can be called an “N95 mask”.
The CDC had released a list of falsely certified masks to alert users, purchasers and manufacturers of the following main scenarios.
- No markings: The mask or package is not clearly marked with the NIOSH approval number (TC).
- NIOSH is misspelled (e.g. NISH, NOISH)
- Other decorative accessories on the mask
- Claimed children’s N95 masks (NIOSH does not approve any children’s masks)
- Earloop masks instead of headgear (currently NIOSH are headgear masks)
The following are some N95 masks that are counterfeit or non-approved by NIOSH.
Certification process
For producers applying for N95 for the first time, the application is divided into two stages.
1 Submit a questionnaire and photos (factory appearance, product line, etc.) to NPPTL for evaluation. After passing the evaluation, NPPTL will give the manufacturer a Three-character Manufacturer’s Code and other related information.
2 After receiving the documents from NPPTL, prepare the detailed application documents. In addition to filling out the standard forms, the company also need to provide the following information to meet the standard requirements.
The official website of the CDC lists the manufacturers of N95
NIOSH-approved respirators have an approval label on the package or inside the package. The certification number can be verified on the NIOSH Certified Equipment List (CEL) or NIOSH Trusted-Source to determine if the respirator has been approved by NIOSH.
Medical N95 mask/respirator
The U.S. Federal Code 21 CFR part 860, according to the different risk levels, the FDA classifies medical devices into three categories with different regulatory approaches.
| Class Ⅰ | Class Ⅱ | Class Ⅲ |
| Lower risk levels, including toothbrush, dental floss, first aid bandages, etc. | Medium risk, Including isolation clothing, medical masks, etc. | Highest risk level, including artificial heart, etc. |
| General controls | Special controls | Premarket approval |
The FDA classifies more than 1,700 different types of devices into 16 medical specialty groups. To determine the classification of devices, follow the 16 medical specialty groups to find the corresponding classification of devices.
Careful comparison of device names reveals that there are a total of seven related product codes, namely: FXX, OXZ, OUK, MSH, ONT, NZJ and ORW, all belonging to Class II medical devices. MSH refers to surgical respirators and specifically refers to medical N95 masks. The specific classification information is summarized in the following table for your reference.
| PC | Device | Regulation number | Type | Submission |
| FXX | Mask, Surgical | 878.4040 | Ⅱ | 510 (K) |
| OXZ | Pediatric/Child Facemask | 878.4040 | Ⅱ | 510 (K) |
| OUK | Surgical Mask with Antimicrobial/Antiviral Agent | 878.4040 | Ⅱ | 510 (K) |
| MSH | Respirator, Surgical | 878.4040 | Ⅱ | 510 (K) Exemption |
| ONT | N95 Respirator with Antimicrobial/Antiviral Agent | 878.4040 | Ⅱ | 510 (K) |
| NZJ | Respirator, N95, for use by the general public in public health medical emergencies | 880.6260 | Ⅱ | 510 (K) |
| ORW | N95 respirator with antimicrobial/antiviral agent for use by the general public in public health medical emergencies | 880.6260 | Ⅱ | 510 (K) |
Note: If the intended use of the N95 mask is to prevent a specific disease or infection, to filter viruses or bacteria, or to reduce/kill microorganisms, it is not covered by the exemption.
Exemptions
In May 2018, the FDA issued a new rule exempting the vast majority of 510(k) applications for N95 masks. This move was made to streamline the N95 approval process for N95 masks only. For other surgical masks, a 510(k) application is still required.
Medical N95 masks
For medical N95 masks, in November 2017, the FDA and CDC/NIOSH signed a memorandum of understanding (MOU) that established a flow chart for the evaluation of N95 masks.
The N95 mask manufacturer first applies to NIOSH, which evaluates whether it is within the scope of the certification, and if it is outside the scope of the certification, it is forwarded to the FDA for evaluation. The FDA also reviews other aspects of product performance, it includes but is not limited to: bacterial/viral filtration efficiency, other biocompatibility assessments such as gas pathway safety assessments, toxicology, etc.
Simply put, a medical N95 mask is a compound.
You can check the official website of the FDA to obtain information on manufacturers qualified to produce N95 masks.The U.S. CDC lists the characteristics of three types of masks
| Medical surgical masks | N95 masks | Medical N95 masks | |
| Evaluation Test Certification | Reviewed by FDA, based on ASTM F2100 standard, requires certain filtration efficiency, breathing resistance, water resistance and flammability. | Must meet NIOSH requirements for minimum filtration efficiency and breathing resistance, evaluated, tested and certified by NIOSH. | By NIOSH for filtration efficiency and breathing resistance, by FDA for water resistance and flammability of the mask. |
| Application Purpose | Prevents the release of potential contaminants from the user into the environment and also protects the wearer from large droplets, sprays and splashes of body fluids. For blocking liquid splashes, droplets and spitting. | Special occupational use to reduce the wearer’s exposure to small particle aerosols and large liquid droplets for any occupational environment requiring N95 protection. For blocking airborne particulate matter from aerosols and viruses. | Used in the medical industry where sterile areas need to be maintained, to reduce the wearer’s exposure to certain airborne particles, sickness blocking splashes and sprays. For blocking airborne particles, droplets and sprays, blocks aerosols and viruses. |
A mask that meets both NIOSH and FDA standards is the medical N95 mask. This type of mask has two functions, it can prevent droplet transmission and airborne diseases, and it can block blood and body fluid spurts that may occur during surgery.
Medical N95 mask marking
- NIOSH-approved manufacturer’s name or registered trademark.
- Capitalized NIOSH letters.
- NIOSH testing and certification number, e.g. TC-84-XXXX
- NIOSH filtration characteristics and filtration efficiency rating
- Mask type
- Batch No: LOT
- SURGICAL MASK logo for use in the operating room
Related assessments information query link: https://www.cdc.gov/niosh/npptl/respirators/testing/default.html
 |
| kn95/ffp mask making machine for sale |




















评论
发表评论