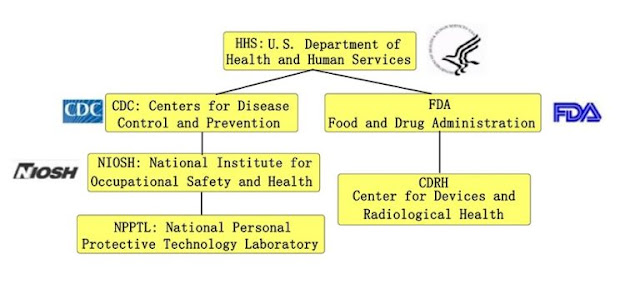
Since the COVID-19, many companies and the populace are concerned about N95 masks, and this article talks to you about the certification of N95 masks. First, let’s take a look at the U.S. regulatory body for masks (respirators). In simple terms, NIOSH is responsible for the certification of personal protective respirator N95, FDA is responsible for the registration audit of medical masks, and medical N95 masks are certified and registered by two agencies. NIOSH Certification Authority N95 is called Respirator in English, now it is called N95 mask in COVID-19, which is actually very different from ordinary masks. The National Institute for Occupational Safety and Health (NIOSH) and the National Personal Protective Technology Laboratory (NPPTL) are the agencies specifically responsible for respirator product certification. NRP Series According to the US Federal Code 42 CFR part 84, NIOSH classifies anti-particle masks into 3 series of 9 categories. Classification N se...